Abstract
BACKGROUND. Anticardiolipin antibodies are immunoglobulins that are measured by enzyme-linked immunosorbent assays using cardiolipin as the antigen. Most of these antibodies are directed towards conformational epitopes of proteins bound to cardiolipin and other anionic phospholipids, the most common being β2-glycoprotein I (β2-Gp1). The clinical importance of the antiphospholipid antibodies is their association with thrombosis: arterial, venous, embolic, and microvascular. While large number of hypotheses have been put forward, the precise mechanism of thrombosis is not known partly because the physiological function of β2-Gp1 (conserved evolutionarily from invertebrates to humans is not known.
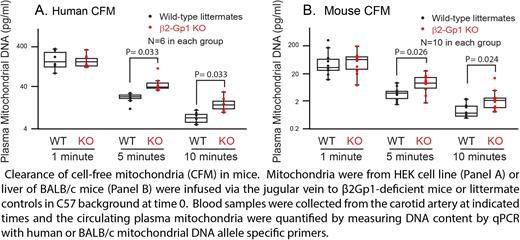
METHODS. We examined the binding of β2-Gp1 to cell-free mitochondria isolated from blood. To assess the physiological effect of β2-Gp1 binding to mitochondria, we performed phagocytosis assays with mouse peritoneal macrophages. We isolated mitochondria from the liver of mito::mKate2 mice, which express bright red fluorescent mitochondria. We quantified the phagocytosis by flowcytometry and immunofluorescence microcopy. We performed similar assays in human macrophages derived from THP-1 cells (a human monocytic cell line) stimulated with phorbol 12-myristate 13-acetate (150 ng/ml) for 3 days. We generate β2-Gp1-deficient mice and measured the circulating mitochondria and the clearance of exogenously infused mitochondria. To determine the survival time of mouse mitochondria in mice, we used polymorphisms of mtDNA of BALB/c mice that differ from the C57BL mice at position 9461 in the ND3 gene (C9461T) and position 9348 in the COIII of mtDNA (A9348G). Allele-specific primers for each strain of mice with the mismatch at the 3' end was used in PCR reactions.
RESULTS. β2-Gp1 binds to mitochondria with maximal binding at physiological concentration of 50-100 μg/ml. The binding markedly increases the uptake of mitochondria by the macrophages reaching a maximum within 30 minutes and at physiological concentration. Cardiolipin is the major anionic phospholipid (>90%) in mitochondria and the binding of β2-Gp1 to mitochondria was inhibited by a cardiolipin binding protein LC3B and by the anionic phospholipid binding protein annexin A5. In addition, the binding of cardiolipin-specific probe NAO (Nonyl acridine orange) was inhibited by β2-Gp1. Furthermore, rotenone, an inhibitor of complex I of the mitochondrial respiratory chain, induces externalization of cardiolipin. Both β2-Gp1 and NAO binding to mitochondria increased following rotenone treatment. These results are consistent with β2-Gp1 binding to cardiolipin in mitochondria. The engulfed mitochondria are delivered to lysosomes. In β2-Gp1-deficient mice, cell-free mitochondria are increased (69 ± 34 pg/ml versus 20.0 ± 8.6 pg/ml in wild-type littermates, P=0.0012). Exogenously infused cell-free mitochondria had a short circulation time of less than 10 minutes in mice. In β2-Gp1-deficient mice, clearance of mitochondria is impaired. Following infusion of isolated mitochondria, β2Gp1-deficient mice had significantly higher levels of transfused mitochondria at 5 minutes (9.9 ± 6.4 pg/ml versus 4.0 ± 2.3 pg/ml in wildtype, p= 0.01) and at 10 minutes (3.0 ± 3.6 pg/ml versus 1.0 ± 0.06 pg/ml in wild-type, p= 0.033, n=10).
CONCLUSIONS. These results suggest the evolutionarily conserved β2-Gp1 performs immunomodulatory and antithrombotic functions by clearing cardiolipin-expressing mitochondria from the circulation.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal